Topic 3: Getting Enough Portable Battery Life
Since a battery represents a portable energy source for many applications, how long a battery will last in an application becomes critical to the user. The more energy stored in a battery, or within a single charging, the longer an application can last without replacement or recharging. That seems pretty obvious. Since one is often transporting their application with them, (i.e., flashlight, computer, e-cigarette, or vaporizer) having sufficient energy often means: leaving one’s home base, venturing for some period of time, using the battery demanding product, and returning to home base for recharging.
Amperage Determination Using Ohm’s Law
Calculations regarding such energy usage is embodied in the frequently used (but never over-used) Ohm’s Law, relating voltage, resistance (in ohms) and current (in amps), and also total energy (in watts). Volts equals Amps times Resistance. (V=IR). In this discussion, the ore important formulation of this is I=V/R or Amperage equals Volts divided by Resistance. This latter is important because it is the amperage that does the work the battery is supposed to do in its particular application (and creates heat).
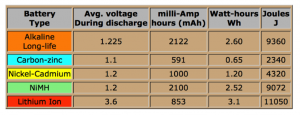
The modern day flashlight may need in the range of 1.5 amps (or 1500 milleamp. If you use it for an hour continuously, it would requirein theory 1500 milleamp-hour (mAH). Based on the AA size table seen in Figure 1, below, one can see how long that flashlight would last for different types of batteries. For only two of the styles would the flashlight last over one hour of theoretical use (see center column (mAh). It becomes apparent that the Lithion ion and Alkaline alternatives look pretty good. One key difference between the two is that the Lithion ion battery can have the option of being rechargeable, while the alkaline is generally thought not to be rechargeable.
Looking at these lower amperage batteries might be fine for a flashlight or computer that uses this smaller amount of amperage, but new applications can require much higher energy demands than the standard AA battery!
Figure 1: Type AA Battery vs. Energy Available
Courtesy of Batteryuniversity.com
The Need for More Energy – Faster!
Because of the highly publicized fires resulting from E-cigarette and vaporizer devices, I am using these topics as illustrative applications.
With the coming of E-cigarettes, vaporizers, hover boards and electric skateboards, for example, comes the usage of much higher amperage requirements at minimum weights. This has driven the manufacturers and marketers towards the characteristics that lithium based batteries can provide. That advantage is stated in terms of how many watt-hours can be stuffed into a certain weight of battery, known as Watt-Hours per Kilogram (wH/kg).
The second chart, also provided by Batteryuniversity.com, is extremely informative in indicating just how much more energy per kilogram of weight the lithiom alternatives provide. The right end of the chart represents several different types of lithiom batteries which give 5-6 times more energy per unit weight than the traditional Lead Acid car type battery on the far left end. This in combination with its recharge ability makes lithium quite desirable.
But there is one more advantage to lithium beyond what these two figures suggest. Lithium has the capability of discharging its stored energy at very high rates, meaning that it can deliver higher amperage amounts per second of use. In the newer applications mentioned above, this higher rate of discharge makes some of the applications themselves possible, where previously, they were not possible with a compact portable energy source.
As an example, in utilizing e-cigarettes or “vaping” type devices, there is a desire by some to utilize very high amperage deliveries, in the range of 20-30 amps (not mille-amps, actual amps). There are writings that describe the relationship among amps, vaporizing temperature, “juice” differences, and flavor. See “sub-ohm vaping” internet articles. They are called “sub-Ohm” since the lower the Ohm resistance in a system, the higher the Amperage energy.
These direct current (DC) amps are not the same in energy amount that you might be familiar in your home, where you have 15 & 20 amp fuses and circuit breakers. A 20-30 amp DC would equal about 1.5 amps in alternating current terms (AC), or about the electricity being utilized by the old 150 watt incandescent light bulb. Nevertheless, This amount of current running stray in your dwelling could still start a house fire. When you consider this amount being used with e-cigarettes or vaporizers to heat the vaporizer coils and “juice”, you have the presence of a hazard which needs to be carefully appreciated and controlled. Strangely, it is not the heating of the coil which is usually the problem, but the current being internally directed within the battery cells by fault to heat the cells rather than just the coil.
Figure 2 below from the battery university website “…compares the specific energy of lead-, nickel- and lithium-based systems. While Li-aluminum (NCA) is the clear winner by storing more capacity than other systems, this only applies to specific energy. In terms of specific power and thermal stability, Li-manganese (LMO) and Li-phosphate (LFP) are superior. Li-titanate (LTO) may have low capacity but this chemistry outlives most other batteries in terms of life span and also has the best cold temperature performance. Moving towards the electric powertrain, safety and cycle life will gain dominance over capacity. (LCO stands for Li-cobalt, the original Li-ion.)”
Figure 2: Typical specific energy of lead-, nickel- and lithium-based batteries
Courtesy of Cadex